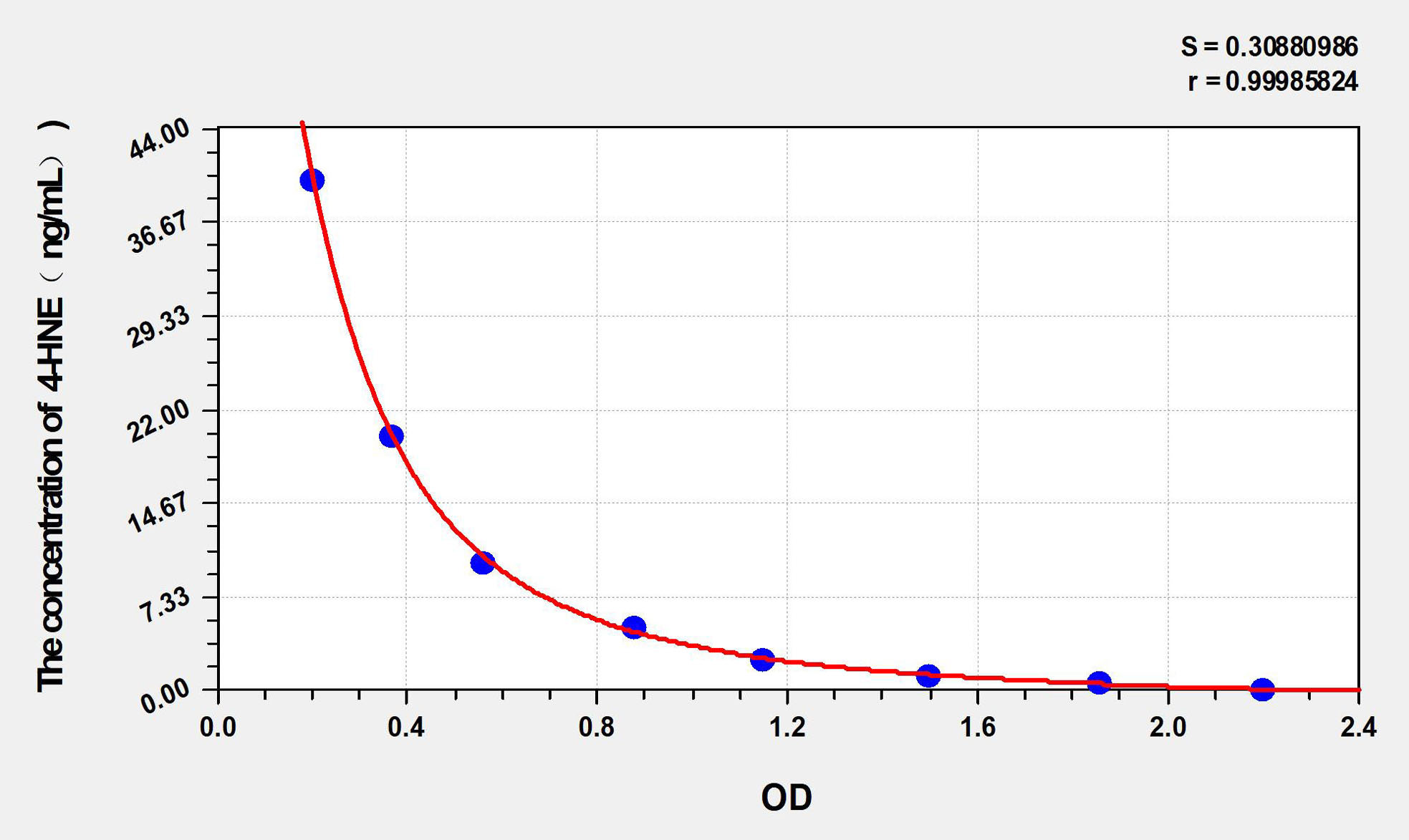
- Product Information
- Components
- Test Principal
- Sample Collection
- Reagent Preparation
- Assay Procedure
- Troubleshooting
| SKU: | EK248919 |
| Category: | Elisa Kits |
| Species: | General |
| Measurement: | Competitive Inhibition |
| Sample Type: | serum, plasma, tissue homogenates, cell lysates, cell culture supernates and other biological fluids |
| Sensitivity: | 0.38 ng/mL |
| Standard: | 40 ng/mL |
| Detection Range: | 0.63-40 ng/mL |
| Assay Time: | 2h |
| Research Area: | Signal transduction;Metabolic pathway;Infection immunity;Hormone metabolism;Dermatology; |
No data available for this product.
No data available for this product.
No data available for this product.
No data available for this product.
No data available for this product.
Troubleshoot Common Elisa Issues
Pipetting and washing techniques for ELISA
Pipetting technique
- Use the correct pipette that is within the range suggested by manufacturer
- Confirm tip is firmly seated on the pipette
- Confirm there are no air bubbles while pipetting
- Change tips between each standard, sample, or reagent
- Use different reservoirs for each reagent
- Pipette sample into the side of wells to avoid splashing
- Always run samples/standards in replicate
- Completely aspirate liquid from all wells by gently lowering an aspiration tip into the bottom of each well.
Note: Take care not to scratch the inside of the well. - Fill the wells with at least 500 µL of diluted wash buffer
- Let soak for 25 to 30 seconds
- Aspirate wash buffer from wells
- Repeat as directed in protocol (usually 4-5 times)
- After washing is complete, invert plate and tap (forcefully, if necessary) dry on absorbent tissue. Be sure to remove any residual liquid.
- Alternatively, a squirt bottle or automated plate washer may be used. Be sure to follow the above.
Problem: No color plate
| Possible Cause | Solution |
|---|---|
| Mixed use of component reagents | Please read labels clearly when preparing or using |
| In the process of plate washing and sample addition, the enzyme marker is contaminated and inactivated, and loses its ability to catalyze the color developing agent | Confirm that the container holding the ELISA plate does not contain enzyme inhibitors (such as NaN3, etc.), and confirm that the container for preparing the Wash Solution has been washed. |
| Missing a reagent or a step | Review the manual in detail and strictly follow the operating steps. |
Problem: Light Color
| Possible Cause | Solution |
|---|---|
| The sample uses NaN3 preservative, which inhibits the reaction of the enzyme | Samples cannot use NaN3 |
| The sample to be tested may not contain strong positive samples, so the result may be normal | In case of doubt, please test again. |
| Wrong filter used for absorbance reading | When TMB is used as the substrate, the absorbance should be read at 450 nm. |
| Insufficient incubation time | Make sure to follow recommended incubation times. |
| Insufficient color reaction | Usually 15 - 30 minutes |
| The number of washings increases, and the dilution ratio of the concentrated lotion does not meet the requirements | Reduce the impact of washing, dilute the concentrated lotion and washing time according to the manual, and accurately record the washing times and dosage. |
| Distilled water quality problem | The prepared lotion must be tested to see if the pH value is neutral. |
| In the process of plate washing and sample addition, the enzyme marker is contaminated and inactivated, and loses its ability to catalyze the color developing agent. | Confirm that the container holding the ELISA plate does not contain enzyme inhibitors (such as NaN3, etc.), confirm that the container for preparing the Washing Solution has been washed, and confirm that the purified water for preparing the Washing Solution meets the requirements and is not contaminated. |
| The kit has expired or been improperly stored | Please use it within the expiration and store it in accordance with the storage conditions recommended in the manual to avoid contamination. |
| Reagents and samples are not equilibrated before use | All reagents and samples should be equilibrated at room temperature for about 30 minutes. |
| Insufficient suction volume of the pipette, too fast discharge of pipetting suction, too much liquid hanging on the inner wall of the tip or the inner wall is not clean. | To calibrate the pipette, the tips should be matched, each time the tips should fit tightly, the pipetting should not be too fast, and the discharge should be complete. The inner wall of the tips should be clean, and it is best to use it once. |
| Incubation temperature constant temperature effect is not good | Keep the temperature constant to avoid the local temperature being too high or too low |
| When adding liquid, too much remains on the medial wall of wells | When adding liquid, the tip should try to add liquid along the bottom of the medial wall of wells without touching the bottom of the hole. |
| Reuse of consumables | The tips should be replaced when different reagents are drawn, and different storage vessels should be used when configuring different reagent components. |
| The bottom of the microwell is scratched or there is dirt | During incubations, cover assay plates with plate sealers. Use a fresh sealer each time the plate is opened. This will prevent wells from contaminating each other. |
| Cross-contamination during sample addition | Try to avoid cross-contamination when adding samples |
| Cross-contamination from manual plate washing | When washing the plates by hand, the first 3 injections of the lotion should be discarded immediately, and the soaking time should be set for the next few times to reduce cross-contamination. |
| Cross-contamination when clapping | Use a suitable absorbent paper towel when clapping the plate, do not pat irrelevant substances into the well of the plate, and try not to pat in the same position to avoid cross-contamination. |
| The liquid filling head of the plate washer is blocked, resulting in unsatisfactory liquid addition or large residual amount of liquid suction, resulting in the color of plate is chaotic and irregular. | Unblock the liquid addition head, so that each well is filled with washing liquid when washing the plate and the residual amount should be small when aspirating liquid. |
| Incomplete centrifugation of the sample, resulting in coagulation in the reaction well or interference of sediment or residual cellular components | Serum plasma should be fully centrifuged at 3000 rpm for more than 6 minutes |
| The sample is stored for too long time, resulting in contamination. | Samples should be kept fresh or stored at low temperature to prevent contamination |
| Incorrect preparation of Washing Solution or direct misuse of concentrated Washing Solution | Please configure according to the manual |
Weak or no signal in ELISA
| Possible Cause | Solution |
|---|---|
| Reagents not at room temperature at start of assay | It is recommended that all reagents be at room temperature before starting the assay. Allow reagents to sit on bench for 15–20 minutes to reach room temperature. |
| Incorrect storage of components | Double check storage conditions on kit label. Most kits need to be stored at 2–8oC. |
| Expired reagents | Confirm expiration dates on all reagents. Do not use reagents that are past the expiration date. |
| Reagents added/prepared incorrectly | Check protocol, ensure reagents were added in the proper order and prepared to correct dilution. |
| Incorrect dilutions prepared | Check pipetting technique—see below—and double check calculations. |
| Capture antibody didn’t bind to plate | If using a ready-to-use kit: Manufactured kits come with plates pre-coated with capture antibody. |
| If coating your own plate with an Antibody Pair Kit: Ensure that you are using an ELISA plate, not a tissue culture plate. Dilute antibody in PBS. Ensure correct preparation and incubation time for both coating and blocking steps. | |
| Wells scratched with pipette or washing tips | Use caution when dispensing and aspirating into and out of wells. Automated plate washers may need to be calibrated so tips don’t touch bottom of wells. |
| Plate read at incorrect wavelength | Make sure to use recommended wavelength/filter. |
Too much signal in ELISA
| Possible Cause | Solution |
|---|---|
| Insufficient washing | Use appropriate washing procedure—see below. At the end of each washing step, invert plate on absorbent tissue and allow to completely drain, tapping forcefully if necessary to remove any residual fluid. |
| Plate sealers not used or reused | During incubations, cover assay plates with plate sealers. Use a fresh sealer each time the plate is opened. This will prevent wells from contaminating each other. |
| Incorrect dilutions prepared | Check pipetting technique—see below—and double-check calculations. |
| Longer incubation times thanrecommended | Make sure to follow recommended incubation times. |
Problem: High background in ELISA
| Possible Cause | Solution |
|---|---|
| Insufficient washing | Use appropriate washing procedure—see below. Increasing duration of soak steps may also help. Add 30 seconds each time you let wash buffer soak. At the end of each washing step, invert plate on absorbent tissue and allow to completely drain, tapping forcefully if necessary to remove any residual fluid. |
| Substrateexposed to light prior to use | Ensure substrate isnot exposed to light—store in a dark place. Limit exposure to light whilerunning assay. |
| The yellowingof the whole plate may be caused by wrong addition of other reagents | Check the componentsand lot numbers of the reagents before the experiment, and confirm that allcomponents belong to the corresponding kit. Reagents from differentkits or different lot numbers cannot be mixed. |
| Streptavidin-HRP contaminates the tip and TMB container orpositive control contaminates the Pre-coated Microplate | When absorbingdifferent reagents, the tips should be replaced. When configuring differentreagent components, different storage vessels should be used. Please use apipette during operation. |
| BiotinylatedAntibody or Streptavidin-HRP concentration too high | Checkwhether the concentration calculation is correct or use after furtherdilution. |
| Color development time is too long | Pleasestrictly follow the steps of the manual. |
| Longerincubation times than recommended | Make sure to followrecommended incubation times. |
| Incorrectstandard curve dilutions prepared | Check pipettingtechnique—see below—and double-check calculations. |
| The wrongfilter was used when the absorbance value was read | When TMB is used asthe substrate, the absorbance should be read at 450 nm. |
Problem: High background in ELISA
| Possible Cause | Solution |
|---|---|
| Incorrect standard curve dilutions prepared | Check pipetting technique—see below—and double-check calculations. |
| Capture antibody didn’t bind to plate | Ensure that you are using an ELISA plate, not a tissue culture plate. Dilute antibody in PBS. Ensure correct preparation and incubation time for both coating and blocking steps. |
Problem: Poor replicate data in ELISA
| Possible Cause | Solution |
|---|---|
| Insufficient washing | Use appropriate washing procedure—see below. Increasing duration of soak steps may also help. Add 30 seconds each time you let wash buffer soak. At the end of each washing step, invert plate on absorbent tissue and allow to completely drain, tapping forcefully if necessary to remove any residual fluid. |
| Capture antibody didn’t bind to plate | Ensure that you are using an ELISA plate, not a tissue culture plate. Dilute antibody in PBS. Ensure correct preparation and incubation time for both coating and blocking steps. |
| Plate sealers not used or reused | During incubations, cover assay plates with plate sealers. Use a fresh sealer each time the plate is opened. This will prevent wells from contaminating each other. |
Problem: Inconsistent results assay-to-assay in ELISA
| Possible Cause | Solution |
|---|---|
| Insufficient washing | Use appropriate washing procedure—see below. At the end of each washing step, invert plate on absorbent tissue and allow to completely drain, tapping forcefully if necessary to remove any residual fluid. |
| Inconsistent incubation temperature | Manufactured kits have optimized protocols. Make sure to follow recommended incubation temperatures. Be aware of fluctuations in temperature due to environmental conditions. |
| Plate sealers not used or reused | During incubations, cover assay plates with plate sealers. Use a fresh sealer each time the plate is opened. This will prevent wells from contaminating each other. |
| Incorrect dilutions prepared | Check pipetting technique—see below—and double-check calculations. |
Product Information
| SKU: | EK248919 |
| Category: | Elisa Kits |